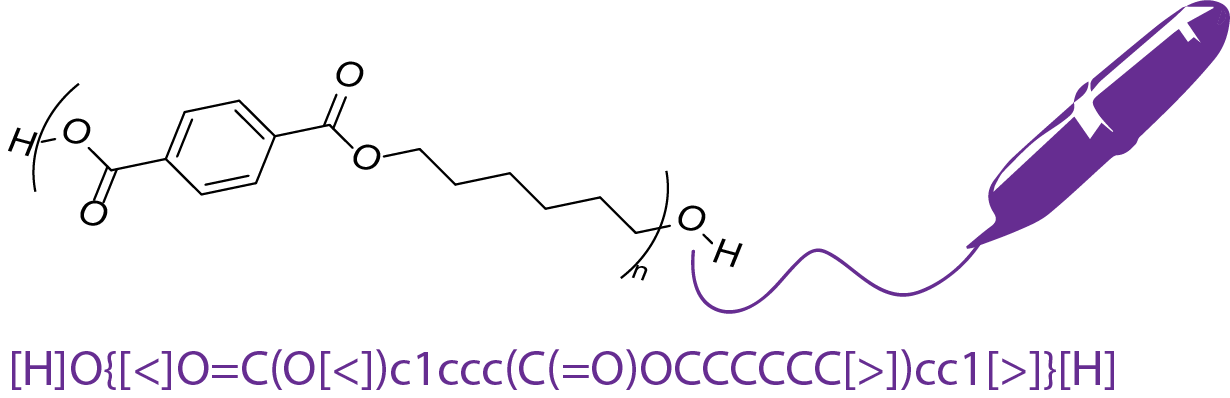Katharina Fransen (Olsen Lab, MIT)
I started graduate school with an interest in polymers and was quickly wrapped up in a project developing high-throughput synthesis and testing and automated experiments for polymer data as a first-year graduate student. While it was a great way to learn synthesis techniques quickly, starting graduate school on a high-throughput synthesis project meant that I was faced with two challenges: keeping track of my many reactions (and reaction attempts) and visualizing what all of those reactions were at the same time.
As anyone who has ever spent time using chemistry, doing research in chemistry, or still has occasional flashbacks to undergraduate chemistry lab knows, this means spending a lot of time on ChemDraw. And while there is something comforting about opening up the ChemDraw ACS 1996 formatted blank document to start a new project, there are also many frustrations that can come with the experience. For example, when opening up the application while writing this, I somehow managed to open 4 instances and the ChemDraw web page. We’ve all experienced the pesky red boxes from bonds that we thought were there, and reached the end of our page when we needed to draw one more molecule. And more than that, everyone who has drawn polymers in ChemDraw understands the difficulty of getting those repeat units to line up just in the right way to make the chemical structure readable. To make things easier, ChemDraw has a great name-to-structure conversion tool which works well for simple monomers, but breaks down for even a simple polymer input like polystyrene:

This left me in a difficult position—as a first-year student, I had synthesized close to 700 polymers—but if I wanted to quickly convey structural features visually, I now had to redraw each of my polymers if I wanted to prepare a presentation, write a paper, or present a poster. After many hours on ChemDraw connecting individual monomers into polymer repeat units, I had a polymer library depicted in ChemDraw, ready for whatever I needed. Except, what happens with CRIPT? Organizing 700 polymers of data is a challenge perfectly suited for CRIPT, but after so much effort translating polymer structures into ChemDraw, would I have to remake them for CRIPT?
CRIPT’s molecular structure drawing tool offers a solution to this problem, allowing for both CRIPT-to-ChemDraw, and ChemDraw-to-CRIPT conversion. Specifically, exporting a ChemDraw figure as MOL Text, allows it to be pasted into the CRIPT Molecular Structure Drawing tool!

After that, a quick addition of an S-Group to specify the polymer repeat type yields your converted structure in addition to the corresponding BigSMILES! Great for when you want to convert your complicated structure to BigSMILES and already have it drawn, or if you’re still mastering the language.

Converting structures back to ChemDraw from CRIPT is just as easy, and super convenient if you have your data and structures stored in CRIPT and need to collect them on one page for making figures. Use the copy molecule button next to the BigSMILES bar.
This is also great for being able to copy structures into other software for analysis—for example, structures can be directly copied out of the CRIPT molecular structure drawing tool and pasted into Mnova for NMR analysis.

As you can see, you can stick with either ChemDraw, switch to the CRIPT structure editor, or work with both depending on your needs without losing features like integration with analysis tools. I recommend testing out both options, figuring out what works for you, and continuing to integrate into the CRIPT ecosystem!